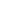Hunterase ICV is indicated for patients with Hunter Syndrome as an enzyme replacement therapy administered intracerebroventricularly.
About
Hunterase ICV
What is
Hunterase ICV?
Composition
Active ingredient: Idursulfase-β 15 mg (1 ml in 1 vial)Indication
Hunterase ICV is indicated for patients with Hunter Syndrome (Mucopolysaccharidosis II, MPS II) as an enzyme replacement therapy (ERT) administered intracerebroventricularly (ICV).Administration
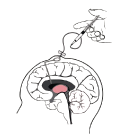
An Implantable cerebrospinal fluid (CSF) reservoir is used to deliver medication to the cerebrospinal fluid (CSF) without performing a spinal tap.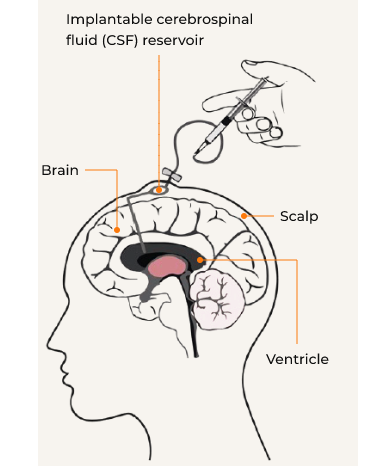
- Administration of Hunterase ICV injection should be considered for patients with MPS II for which improvement of central nervous system (CNS) symptoms is necessary.
- Hunterase ICV should be administered to patients who have received intravenous idursulfase with confirmed tolerability.
- During the phase I/II study, Hunterase ICV was administered and intravenous idursulfase was also continued throughout the study.
How Was Hunterase ICV Developed?
Study history
In Japan, intravenous ERT has been used for more than 120 patients with MPS II.However, intravenously administered Idursulfase cannot penetrate the blood-brain barrier (BBB) and therefore it cannot reach the cerebral parenchyma.
To improve the CNS symptoms of neuronopathic MPS II patients, including cognitive decline, enzyme preparation that can reach the cerebral parenchyma have been sought.
Non-clinical evidence has shown that idursulfase administered intrathecally or intracerebroventricularly (ICV) rapidly reaches the brain.
Hunterase ICV study has been administered by ICV.
ICV ERT can be an effective and safe therapy for treating CNS symptoms, as demonstrated for cerliponase alfa, an approved treatment for neuronal ceroid lipofuscinosis type 2 disease.
From a safety point of view, short-time injection (1 min) and the CSF reservoir were good methods and materials.
Why Should CNS Symptoms Be Considered?
Non-neuronopathic (attenuated) type: Minimal CNS involvement
Common symptoms & signs
Developmental delay
Coarse face
Short stature
Skeletal abnormalities
(dysostosis multiplex)
Coarse face
Short stature
Skeletal abnormalities
(dysostosis multiplex)
Joint contracture
Hepatosplenomegaly
Upper airway obstruction
Valvular heart disease
Hepatosplenomegaly
Upper airway obstruction
Valvular heart disease
Neuronopathic (severe) type: CNS involvement
Neurobehavioral symptoms
Aggression
Hyperactivity
Sleep disturbances
Progressive neurological decline
Cognitive impairment
Hyperactivity
Sleep disturbances
Progressive neurological decline
Cognitive impairment
Clinical Trials
Hunterase ICV (intracerebroventricular idursulfase-β) was well tolerated, reduced the heparan sulfate concentration in the cerebrospinal fluid, and was effective at preventing and stabilizing developmental decline in patients with neuronopathic MPS II.
Phase I/II clinical study aimed to evaluate the efficacy and safety of intracerebroventicular idursulfase-β in patients with mucopolysaccharidosis II (MPS II).
The open-label, phase I/II study was conducted in 6 patients aged 23-65 months with severe MPS II and significant developmental delay using the historical control group at two clinical sites in Japan.
A placebo control was not considered because of an unacceptable risk/benefit ratio as with device placement and ICV administration of an inactive comparator.
All patients had tolerated ≥24 weeks of treatment with intravenous idursulfase before the start of the study.
Intracerebroventricular Idursulfase-β (increasing from 1 to 30 mg between weeks 0 and 24, followed by a 30-mg final dose) was administered once every 4 weeks using an implanted CSF reservoir.
Intravenous administration of idursulfase was also continued throughout the study.
Phase I/II clinical study aimed to evaluate the efficacy and safety of intracerebroventicular idursulfase-β in patients with mucopolysaccharidosis II (MPS II).
The open-label, phase I/II study was conducted in 6 patients aged 23-65 months with severe MPS II and significant developmental delay using the historical control group at two clinical sites in Japan.
A placebo control was not considered because of an unacceptable risk/benefit ratio as with device placement and ICV administration of an inactive comparator.
All patients had tolerated ≥24 weeks of treatment with intravenous idursulfase before the start of the study.
Intracerebroventricular Idursulfase-β (increasing from 1 to 30 mg between weeks 0 and 24, followed by a 30-mg final dose) was administered once every 4 weeks using an implanted CSF reservoir.
Intravenous administration of idursulfase was also continued throughout the study.
Primary endpoint
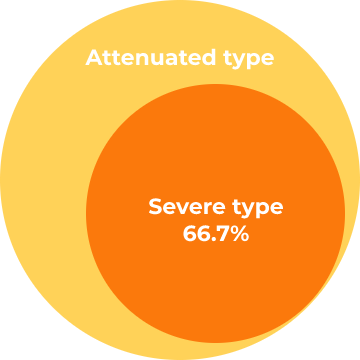
Heparan sulfate (HS) concentration in the cerebrospinal fluid (CSF).Results
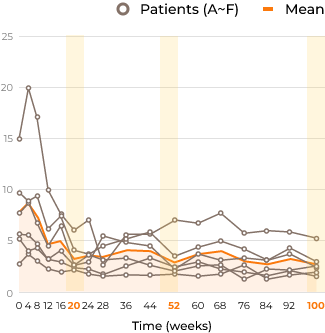
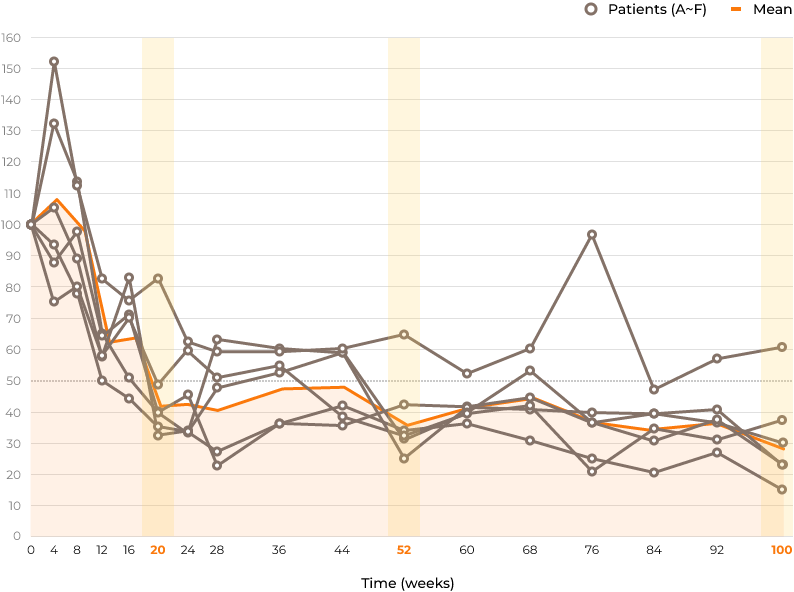
HS concentrations in the CSF decreased (40% - 80%) from baseline to week 100 in all patients following ICV idursulfase-β administration.Change in HS in the CSF of patients with MPS II from the start of idursulfase-β treatment up to week 100
HS concentrations in CSF (µg/mL)
Ratio of each HS concentration relative to baseline in CSF (%)
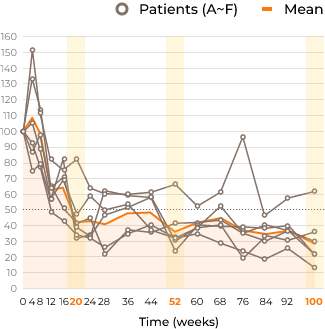
Secondary Endpoints and Results
Secondary endpoints
Developmental age (DA) determined by the Kyoto Scale of Psychological Development 2001 (KSPD) in the following three areas: postural-motor, cognitive-adaptive and language-social (an individualized face-to-face test).Results
Monthly ICV administration of idursulfase-β maintained or increased DA in five of six patients compared with the historical control group receiving intravenous idursulfase.At 100 weeks (about 2 years) after starting this study, six patients who received ICV idursulfase-β had a 5.1-month increase in mean DA compared with 13 historical control patients who received only intravenous administration of idursulfase.
Comparison with the historical control data in patients with MPS II
Change from screening period up to week 100 in DA (all 3 areas) by KSPD (months)
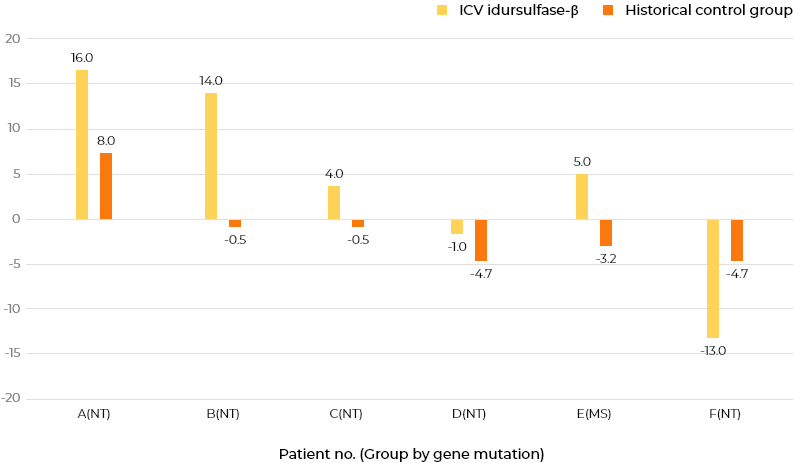
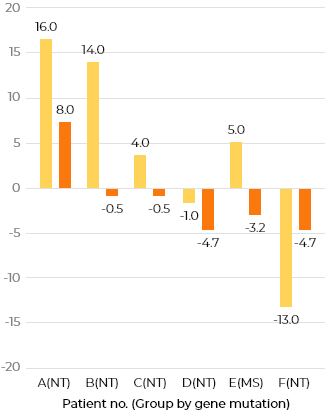
Change difference from screening period up to week 100 in DA (all 3 areas) by KSPD (months)
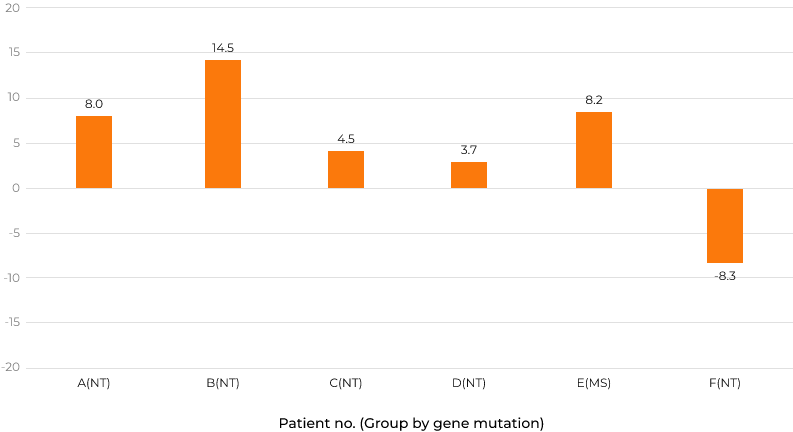
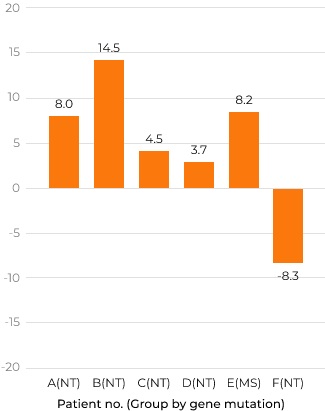
Group MS: characterized by missense mutations and presumed to have slight residual enzyme activity Group NT: considered to have null type mutations, such as deletions, recombination with pseudogene, and nonsense mutations
Safety Information
Precautions
Storage and Shelf-life
They may depend on the approved conditions in individual countries.
- Store at 2 ºC to 8 ºC in a hermetic container.
- The shelf-life of this product is 24 months from the date of manufacture.
ENG Package Insert
How to Use
Injection Procedure
- Implant a CSF reservoir under the patient’s scalp for i.c.v. administration.
- To prevent fluctuations in intraventricular pressure, collect CSF (2 ml) of the same volume with Hunterase ICV (usually 30 mg) to be injected in advance.
- Administer Hunterase ICV without dilution over at least 1 min.
Injection Schedule
Hunterase ICV is intracerebroventricularly administered once every 4 weeks (Hunterase ICV: 15 mg per vial).
Please note that in the Phase I/II trial, intravenous administration of idursulfase (0.5 mg/kg/week) was also continued throughout the study; an interval of ≥24 hours was set between intravenous idursulfase and Hunterase ICV.
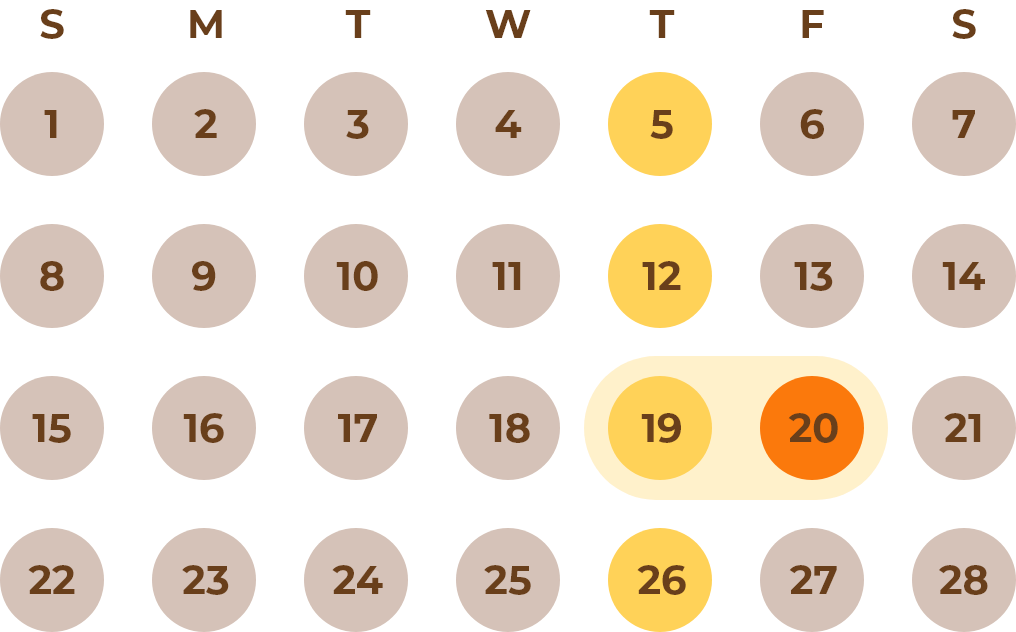

Please note that in the Phase I/II trial, intravenous administration of idursulfase (0.5 mg/kg/week) was also continued throughout the study; an interval of ≥24 hours was set between intravenous idursulfase and Hunterase ICV.
Example

IV : Once a week
ICV : Once a month
A minimum 24-hour interval is recommended between IV and ICV injections.

≥24 H

References
- Orphanet J Rare Dis. 2013;8:42
- Mol Ther Methods Clin Dev. 2021;21:67-75
- Mol Genet Metab. 2015;114(2):156-60